Iron Nitride


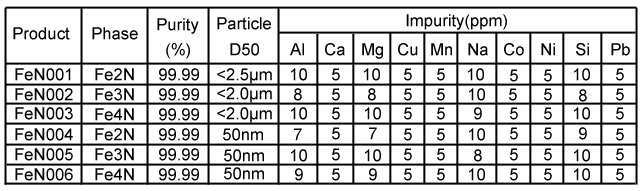

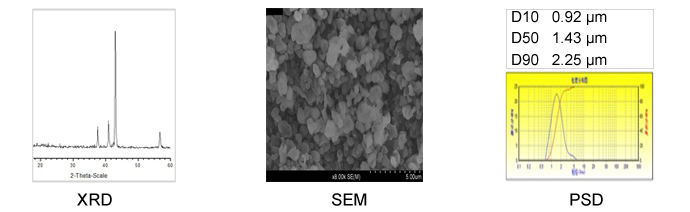

Molecular Formula: FeNx CAS #: 37245-77-5 Color: Grey Solubility in water: Insolubility | Iron has five nitrides observed at ambient conditions, Fe2N, Fe3N4, Fe4N, Fe7N3 and Fe16N2.Iron nitride is easy to decompose at low temperatures(less than 400℃),insoluble in water. But iron nitride can react with water and release ammonia. At high pressure, new nitrogen-rich nitrides (N/Fe ratio equal or greater to one) were discovered. These include the FeN, FeN2 and FeN4 solids which become thermodynamically stable from GPa, respectively. |

◇Iron nitride can be used to prepare the strongest magnets.
◇Colloidal solution of magnetic iron nitride nanoparticles is a way to create ferrofluids.
◇Iron nitrides also make the strongest naturally magnetic material.

- Barium Nitride
- Vanadium Nitride
- Molybdenum Nitride
- Copper Nitride
- Cobalt Nitride
- Titanium Nitride
- Yttrium Nitride
- Terbium Nitride
- Praseodymium Nitride
- Manganese Nitride
- Chromium Nitride
- Gadolinium Nitride
- Dysprosium Nitride
- Erbium Nitride
- Cerium Nitride
- Iron Nitride
- Lanthanum Nitride
- Gallium Nitride
- Ytterbium Nitride
- Calcium Nitride
- Europium Nitride
- Strontium Nitride
- Aluminum Nitride
- Silicon Nitride




